CORRECT ANSWER IS C
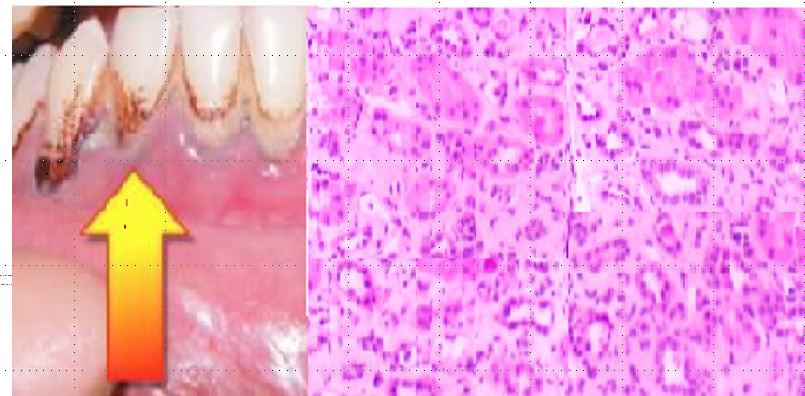
CHRONIC LEAD NEPHROPATHY. Patient has chronic Lead nephropathy which can explain his multiple findings. Lead nephropathy should be considered in the differential diagnosis of patients who present with the CKD, HTN, gout, and evidence of lead poisoning in other organ systems. Picture of his Gums reveals classic Burtons line. Burton’s line is a blue-purplish line on the gums seen in lead poisoning. It is caused by a reaction between circulating lead with sulphur ions released by oral bacterial activity, which deposits lead sulphide at the junction of the teeth and gums. Classic findings of kidney biopsy in lead nephropathy shows chronic interstitial nephritis, nonspecific tubular atrophy, interstitial fibrosis, minimal inflammatory cells. His occupation is ammunition factory worker which has high exposure to lead. Lead nephropathy is a potential complication of prolonged (5 to 30 years), high-level lead exposure (blood lead levels- BLL >60 mcg/dL. Lead nephropathy has been associated with hypertension, increased cardiovascular risk, and chronic gout.
Acute, high-level lead poisoning can present with Fanconi-type syndrome, such as glucosuria, aminoaciduria, and phosphaturia due to decreased in reabsorption due to proximal tubular injury and is associated with intranuclear inclusion bodies in proximal tubular cells. The Fanconi syndrome generally resolves with treatment of lead poisoning, although glucosuria and aminoaciduria may persist for a prolonged period. Acute toxicity symptoms may include abdominal pain (“lead colic”), myalgia, fatigue, decreased sexual libido, headaches, concentration and memory deficits.
Chronic lead nephropathy is due to more prolonged exposure to high level of lead and usually present with CKD, HTN, Minimal proteinuria, a benign urine sediment, and hyperuricemia with gout. The diagnosis of lead nephropathy requires Identification of potential current or past sources of lead exposure, Assessment for the extrarenal signs and symptoms of lead poisoning, assessment by levels of lead in whole blood. chronic lead exposure can also produce anemia, neurocognitive decline, tremor, and hypertension.
Treatment of lead toxicity with chelation therapy is indicated for adult patients with a BLL >80 mcg/dL and also for patients with BLL >50 mcg/dL with symptoms or signs of lead toxicity. Usually chelation therapy is NOT indicated for patients with BLL <50 mcg/dL. Chelation should not be undertaken unless exposure is stopped, as continuing lead exposure with start of chelation may result in enhanced absorption of lead and worsening, rather than improvement in lead toxicity. The two most commonly used chelating agents for adults are DMSA (2,3-dimercaptosuccinic acid, succimer) and calcium disodium ethylenediaminetetraacetic acid (EDTA).
CHRONIC URATE NEPHROPATHY – Chronic urate nephropathy in the absence of tophaceous gout, is a relatively rare condition. Patient is this case does not have tophi. CKD in chronic urate nephropathy is due to deposition of urate crystals in the medullary interstitium resulting in inflammation, interstitial fibrosis and tubular atrophy. A bland urinary sediment, mild proteinuria, and hyperuricemia disproportionate to the degree of impaired kidney function are features of urate nephropathy. Also kidney biopsy did not reveal any urate crystals.
CHRONIC ALLOGRAFT NEPHROPATHY (CAN) should be suspected in any kidney transplant presenting with a slow, progressive loss of kidney function, usually associated with hypertension and worsening proteinuria. Chronic calcineurin inhibitor (CNI) nephrotoxicity is manifested by worsening creatinine (due to abnormalities in tubular function, glomerular and vascular components ) and an increase in blood pressure- Kidney biopsy reveals an obliterative arteriolopathy due to primary endothelial damage), ischemic scarring of the glomeruli, vacuolization of the tubules, global and focal segmental glomerulosclerosis, and focal areas of tubular atrophy and interstitial fibrosis producing a “striped” pattern on low power.Also this patient is not currently taking any drugs that inhibit cytochrome P-450 which can increasing exposure to CNI metabolites or to drugs that inhibit P-glycoprotein-mediated efflux of CNIs from tubular epithelial cells, which can elevate local renal exposure to CNIs
HTN RELATED NEPHROSCLEROSIS can contribute to chronic GN after many years, but it is not the primary cause of renal failure in this patient, but it is likely secondary effect due to his chronic lead exposure.
REFERENCES
Nephropathy in Chronic Lead Poisoning | JAMA Internal Medicine | JAMA Network
Lead Nephropathy, Gout, and Hypertension – ScienceDirect
Uric Acid Nephropathy: Practice Essentials, Pathophysiology, Etiology (medscape.com)